When the term "rare earth elements” (REEs) is used, it typically refers to a group of elements including the lanthanide series (La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu), as well as Sc and Y.
REEs are used extensively in electronics and advanced technologies, which are utilized in: airplanes, automobiles, medical devices, smartphones, television, computers, and camera lenses to name a few. The continual advancement of technologies has increased the demand for REEs, so the search for large deposits of REEs and quality ore material is ongoing.
Raw geological materials containing REEs are typically found in low concentrations. The ability to precisely detect and quantify REEs in geological samples is a critical component of successful geological exploration. This determines the viability of new mining sites, as well as help to expand existing ones.
The common techniques employed to analyze the REEs of geological samples include inductively coupled plasma mass spectrometry (ICP-MS), x-ray fluorescence (XRF), and instrumental neutron activation analyses (INAA). While XRF and INAA have their own advantages and disadvantages, both techniques have poor detection limits when compared to ICP-MS – a vital requirement when detecting low concentrations REEs.
As a result, measuring REEs content by ICP-MS post-sample preparation is the broadest used analytical technique. Using an appropriate sample preparation method – primarily acid digestion or fusion – prior to analysis by ICP-MS is critical in achieving accurate results. A variety of complications can be introduced to the analyzis, depending upon the chosen method of preparation.
The fusion of samples in lithium borate flux is a commonly used preparation technique for matrices that are very refractory, or otherwise hard to digest. In ICP-MS analysis, the high boron and lithium backgrounds in these types of prepared samples can present challenges, which would need addressing. For matrix effects because of the increased amount of total dissolved solids (TDS) in the prepared solution.
In addition, REEs are frequently present in very low concentrations, and many have oxide and isotopic overlaps - these factors require extra considerations. The combination of the All Matrix Solution (AMS, an online argon dilution system)1, second-generation Triple Cone Interface with OmniRing™ technology2, four gas channels with on-line gas mixing, Universal Cell Technology (UCT), and multi-quadrupole design of PerkinElmer’s NexION® 5000 ICP-MS, provides many of the tools needed to overcome these issues.
This demonstration note outlines the analysis of three certified reference materials (CRMs) that contain REEs, prepared by fluxing the samples with lithium borate. The samples contain different ore materials all comprised of REEs, and are analogous to exploration samples.
Experimental
Samples and Sample Preparation
In this application note, certified reference materials (CRMs) were provided by an external laboratory. These CRMs had already undergone lithium metaborate/tetraborate fusion and dissolution in 5% HNO3 (ca. 100 mg of sample, 100 mL dilution). In addition, dilution of 100x was conducted on every CRM before analyzes, using a diluent of 0.5 % HNO3. The CRMs used were REE-1, REE-2, and REE-3 (CanMET Mining, Ottawa, Ontario, Canada).
Blank fusion solutions were also supplied so that calibration blanks and standards could be matrix matched. For all samples, the standard concentrations prepared were 0.2, 2 and 20 ppb. For elements Al, Ca, Fe, K, Mg, Na, and Si, the standard concentrations were 0.2, 2, 20, 200, and 400 ppb. Due to the heavy and complex matrix, 10 ppb rhodium was used as the internal standard and added online through the internal standard port of PerkinElmer's High Throughput System (HTS), a flow injection sample introduction module.
Instrumental Conditions
The analyzes was carried out on a NexION 5000 ICP-MS, equipped with a SMARTintro™ Ultra High Purity quartz torch, a fixed 2.0 mm injector, a SilQ spray chamber, and a PFA nebulizer. The NexION’s All Matrix Solution (AMS) was used for argon-based aerosol dilution, further reducing the matrix suppression resulting from the lithium borate fusion.
In order to aid long-term stability, a PC3 spray chamber chiller was used to study the samples. To elevate throughput through its vacuum-loaded sample loop and flow injection system, the HTS (High Throughput System) module was used. Specific instrument conditions can be found in Table 1.
Table 1. NexION 5000 ICP-MS Instrumental Conditions. Source: PerkinElmer
| Parameter |
Value |
| Plasma Gas Flow |
16 |
| Aux Flow |
1.2 |
| RF Power (W) |
1600 |
| Neb Gas Flow |
0.99 |
| AMS Gas Flow |
0.11 |
| Peltier Cooler Temp |
10 ºC |
| Pump Tubing - Carrier |
Green/Orange |
| Pump Tubing - IS |
Green/Orange |
| Sample Uptake Rate (mL/min) |
0.14 |
To better utilize the interference removal capabilities of the NexION 5000 system multiple cell modes were used. Dynamic bandpass filtering within the quadrupole Universal Cell, m/z selection for the main resolving quadrupoles, and optimization of collision and reaction gas flows were automated through Syngistix™ for ICP-MS software.
The ion guide mode what selected because it delivers the lowest background equivalent concentrations (BECs) for the all the analytes of interest. However, depending on the interferences present on the specific analyte, a combination of gas profiles was utilized (Table 2), providing examples of why multi-quadrupole technology, once paired with multiple gas channels, can offer superior analytical options when compared to less advanced instrumentation.
One important advantage of multi-quadrupole instruments is the ability to mass-shift a target element. This is accomplished by reacting the element with a reactive gas to a higher mass, away from interferences, resulting in the possibility of erroneous results. To form oxides on the target elements, oxygen can be used, shifting 16 amu higher. In contrast, gases like pure ammonia can form cluster ions with certain elements, moving further up the mass range, away from potential interferences.
This series of reactions require active control to prevent run-away chemical reactions from taking place. The customizable bandpass tuning, afforded by the unique quadrupole UCT, allows the users to prevent the formation of reaction byproducts, and their subsequent reaction with the reaction gas or impurities in the gas.
Table 2. Elements Analyzed, Gas Modes, and Quadrupole Settings. Source: PerkinElmer
| Analyte |
Gas Mode |
Isotope Q1/Q3 |
| Al |
Ammonia DRC |
27/27 |
| Ba |
STD |
137/137 |
| Ca |
Ammonia DRC |
40/40 |
| Ce |
Ammonia DRC |
140/140 |
| Co |
Ammonia DRC |
59/59 |
| Cr |
Ammonia DRC |
52/52 |
| Cs |
STD |
133/133 |
| Cu |
Ammonia DRC |
65/65 |
| Dy |
Oxygen DRC |
163/179 |
| Er |
Oxygen DRC |
166/182 |
| Eu |
Oxygen DRC |
153/169 |
| Fe |
Ammonia DRC |
56/56 |
| Gd |
Oxygen DRC |
157/173 |
| Hf |
Oxygen DRC |
178/194 |
| Ho |
Oxygen DRC |
165/181 |
| K |
Ammonia DRC |
39/39 |
| La |
Oxygen DRC |
139/155 |
| Lu |
Oxygen DRC |
175/191 |
| Mg |
Ammonia DRC |
24/24 |
| Mn |
Ammonia DRC |
55/55 |
| Na |
STD |
23/23 |
| Nb |
Ammonia DRC |
93/93 |
| Nd |
STD |
143/143 |
| P |
Oxygen DRC |
31/47 |
| Pb |
STD |
208/208 |
| Pr |
Oxygen DRC |
141/157 |
| Sc |
Oxygen DRC |
45/61 |
| Si |
Ammonia/Hydrogen DRC |
28/28 |
| Sm |
Oxygen DRC |
147/163 |
| Sn |
STD |
118/118 |
| Sr |
Ammonia DRC |
88/88 |
| Tb |
Ammonia DRC |
159/174 |
| Th |
STD |
232/232 |
| Ti |
Ammonia DRC |
48/114 |
| Tm |
Oxygen DRC |
169/185 |
| U |
STD |
238/238 |
| Y |
Oxygen DRC |
89/105 |
| Yb |
Oxygen DRC |
172/188 |
| Zn |
Ammonia DRC |
66/66 |
| Zr |
STD |
90/90 |
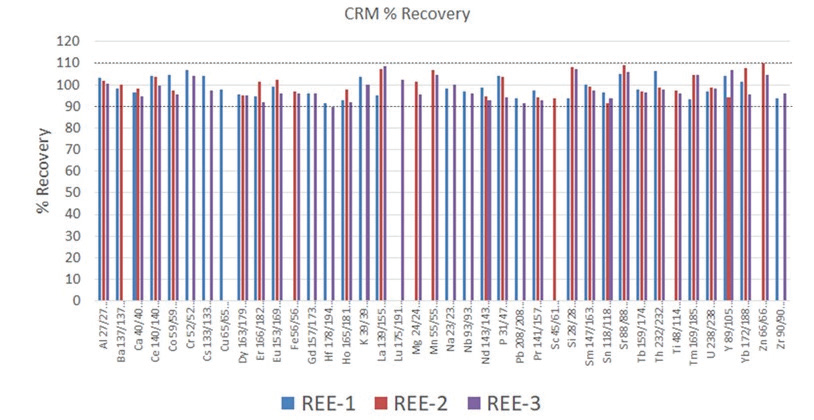
Figure 1. Analyte recoveries in certified reference materials REE-1, REE-2, and REE-3. Not all elements are certified in each reference material. Image Credit: PerkinElmer
Results and Discussion
Accuracy of the methodology was determined by analyzing the three aforementioned reference materials. As seen in Figure 1, 80% of the analyte recoveries are within 5% of the certified values and 100% within 10% of the certified values.
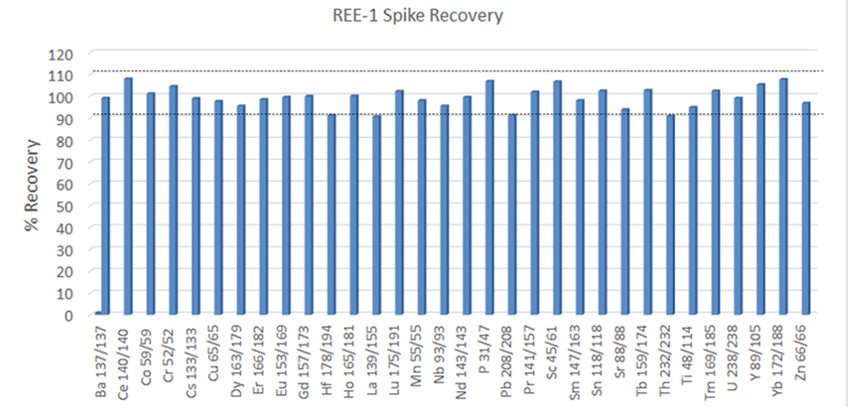
Figure 2. A 4 ppb spike was performed on the CRM REE-1. This spike level was too low for a proper spike recovery test on Al, Ca, Fe, K, Mg, Na, Si, and Zr in this CRM. Image Credit: PerkinElmer
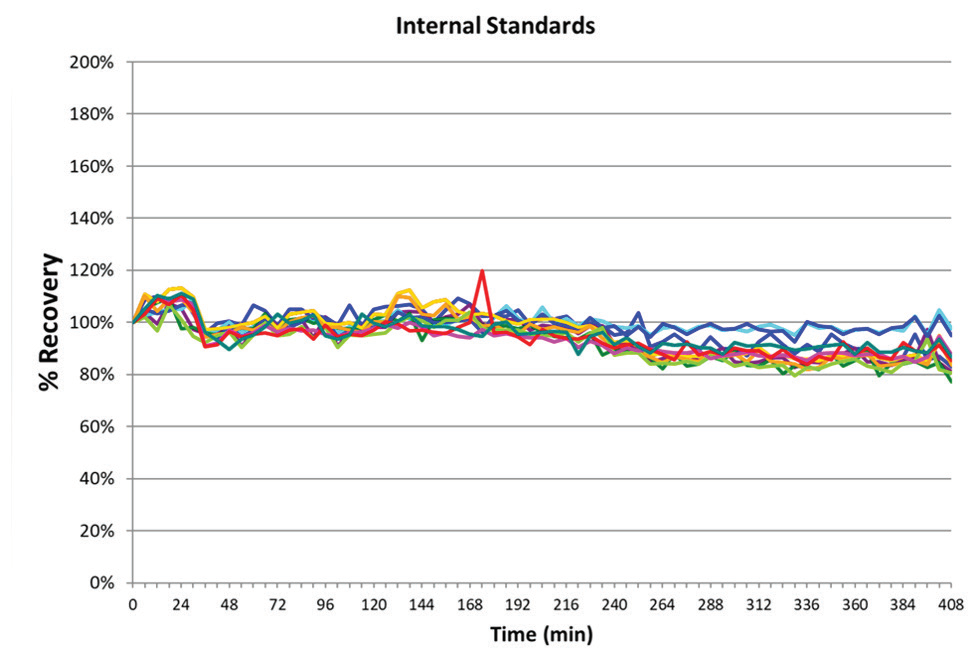
Figure 3. Long-term stability based on internal standard (Rh) measured over a nearly 7-hour run with multiple gas modes. Image Credit: PerkinElmer
Of particular interest are the conditions used for Si and P; these are an example of the flexibility of the Universal Cell’s reaction capability. For Si, ammonia and hydrogen are combined online in the cell for the best interference removal of N2 and CO on mass 28. On the other hand, P is monoisotopic, with 100% isotopic abundance at 31 amu. However, there can be interferences from N and Si on this mass (NOH, SiH, NO), which will make the analysis more challenging for single quadrupole instruments. Although, with multi-quadrupole ICP-MS technology, P is mass shifted to PO and examined clear of these interferences, providing excellent CRM recoveries (Figures 1 and 2, respectively).
Results of a spike recovery test (4 ppb spike, Figure 2) conducted on REE-1 as an additional test of accuracy, where all elements were recovered within 10% of the expected results. As the heavier REE elements are often plagued by interferences from the lower mass REE elements' oxides, the majority of these elements were measured using Mass Shift mode with oxygen as a reaction gas in the cell.
This takes advantage of these elements' propensity, producing oxides quickly in a kinetically favorable reaction, and mass shifts them away from interferences (Table 2). The spike recoveries for the REE elements are near 100%, demonstrating that the interferences have been effectively removed. Had interference not been removed, higher than expected recoveries for higher-mass REE elements from Sm to Lu would have been observed.
Long-term stability was confirmed during the seven-hour analysis of the lithium borate fused samples. The analytical run included multiple fusions of each CRM, repeated in sequence, in addition to the matrix blanks and simulated samples in the same fusion matrix. This demonstrated the effect of analyzing samples with high total dissolved solids (TDS) on the sample introduction, and internal components of the NexION 5000 ICP-MS.
The internal standard recoveries relative to the calibration blank were measured, and are detailed in Figure 3. All internal standards recovered within 20% of their values being standardized to their response within the calibration blank, further confirming the validity of the method's robustness, and analytical instrumentation.
This exceptional performance is a direct result of the NexION 5000's instrumental design considerations, including its solid-state, free-running RF generator3, Triple Cone Interface2, and Quadrupole Ion Deflector. Utilizing AMS also aids long-term stability through an argon dilution effect, reducing matrix build-up on the cone system.
Conclusion
The analyses outlined within this application note demonstrates the accurate and highly stable quantification of REEs, and other elements of interest, in geological samples. The NexION 5000 Multi- Quadrupole ICP-MS delivers outstanding robustness, even for sample types that normally present analytical challenges – this is due to AMS and the combination of the Triple Cone Interface with OmniRing technology.
In addition, superior interference was removed on all analytes of interest. In many instances, Multi Quad mode was able to ensure control of the ions that enter the cell, and the reactions within the cell. Finally, having four gas channels yields maximum specificity in the reaction chemistry, allowing the most effective single or mixed reaction gas to be used without any compromise.
Acknowledgements
Produced from material originally authored by Andrew Rams, Senior Field Application Scientist, PerkinElmer Inc. (Woodbridge, ON, Canada)
References
- All Matrix Solution System for NexION ICP-MS Platforms, PerkinElmer Technical Note, 2017.
- Advantages of a Novel Interface Design for NexION 5000 ICP-MS, PerkinElmer Technical Note, 2020.
- Advantages of a Novel Plasma Generator for the NexION 1000/2000/5000 ICP-MS Systems, PerkinElmer Technical Note, 2020.
Consumables Used
Table 3. Source: PerkinElmer
| Component/Description |
Part Number |
| PFA ST3 Nebulizer with Integrated Gas Line |
N8152378 |
| Fixed 2.0 mm Injector UHP Quartz Torch |
N8152428 |
| SilQ Spray Chamber with AMS Gas Port |
N8152539 |
Grey/Grey 1.30 mm I.D.
Santoprene Peristaltic Pump Tubing |
N8152415 |
| Orange/Green Flared 2-Stop PVC Pump Tubing |
N8145197 |
| Gas Line- Matrix |
N8152374 |

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer.
For more information on this source, please visit PerkinElmer.